Purified Vero Cell Rabies Vaccine Validity, Intradermal Post Exposure Rabies Vaccination With Purified Vero Cell Rabies Vaccine Comparison Of A One Week 4 Site Regimen Versus Updated Thai Red Cross Regimen In A Randomized Non Inferiority Trial In The Philippines Sciencedirect
All subjects who received PrEP with CPRV had protective neutralizing antibody Nab titers 05 IUml 14 days after completing vaccination. In a pre-exposure setting in this study.

Purified Vero Cell Rabies Vaccine Packaging Type Paper Box For Clinical Rs 290 Pack Id 14090049262
These are inactivated by ß-propriolactone and purified by ultracentrifugation Jaiiaroensup et al 1998.

Purified vero cell rabies vaccine validity. Purified Vero cell rabies vaccine PVRV is a new effective but inexpensive tissue culture rabies vaccine for human use. Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after. Purified Vero cell rabies vaccine was used to treat 19 patients who experienced fox bite.
Antibody responses to a conventional rabies preexposure regimen of a new purified Vero cell rabies vaccine PVRV and a human diploid cell vaccine HDCV were compared in 80 healthy Kenyan veterinary students. Safety and efficacy of purified vero cell rabies vaccine given intramuscularly and intradermally. Results of a prospective randomized trial.
The protective effect of a new potentially economical tissue-culture rabies vaccine purified vero-cell rabies vaccine PVRV was tested in 106 patients bitten by animals with proven rabies. In addition the residual DNA from Vero cells has been proofed positive to carcinogenicity test after over 170 passages thus their genomic DNA carried in vaccines could be oncogenic and hence poses a potential risk for human carcinogenesis 6 7 and 8 therefore strictly removal of Vero cell DNA was needed. Seventeen patients survived and 2 patients died of rabies.
COVID-19 Vaccine Vero Cell Inactivated detailed edition Created Date. In PEP study Nab titers in the CPRV groups reached 05 IUml in all subjects by day 14 through day 90 after. The second shot is usually given 7 days after the first followed by a third shot 2 or 3 weeks later.
This regimen will be applied as simulated PEP regimen to healthy subjects ie. Reference vaccine purified vero cell vaccine. To demonstrate that Purified Vero Rabies Vaccine - Serum Free Vaccine generation 2 VRVg-2 is non-inferior to Verorab and Imovax Rabies vaccines when co-administered with human rabies immunoglobulin HRIG in terms of proportion of subjects achieving a rabies virus neutralizing antibody RVNA titer 05 IUmL at D28 ie 14 days after the fourth vaccine injection.
Listing a study does not mean it has been evaluated by the US. Evolving rabies vaccination trends including shorter intradermal ID regimens with reduced volume along with WHO recommendation for ID administration has driven recent ID PVRV regimen assessments. 2-2-2-0-2 with a new chromatographically purified Vero-cell rabies vaccine CPRV is lacking.
Thirty-nine seronegative patients age range 2-14 years with rabies. A comparison with placebo combined with each rabies vaccine was included to differentiate between the contribution of immune globulin and vaccine and to investigate potential interactions between immune globulin and vaccine. Forty-three of the students received the PVRV and 37 received the HDCV on days 0 7 and 28.
RVNA provided by CL184 or HRIG as well as vaccination response. Immunogenicity and Safety of a Purified Vero Rabies Vaccine The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says.
Vero cell covid vaccine efficacy rate. The subjects received conventional PrEP with CPRV or PVRV in PrEP study or received intradermal PEP with CPRV or PVRV and rabies immunoglobulin in PEP study. Fifty-eight subjects with low-risk exposure to rabies were randomized into 4 groups to receive full-dose 05 ml intramuscular injection of PVRV on days 0 3 7 14 and 28 or 4 2 or 1 intradermal.
Results from a randomized controlled phase-II trial PVRV-NG was shown to be at least as immunogenic as Verorab and to present a similar safety profile. For pre-exposure prevention of rabies you will need to receive a total of 3 shots. Purified Vero cell rabies vaccine is safe carries a very low adverse reaction rate and is effective in preventing rabies in severely exposed subjects when used with human or equine rabies immune globulin.
SPEEDAchromatography purified vero cell derived rabies vaccineapproved by Thai FDA on 8 April 2009 and drug registration code of SPEEDA is 1C 9002251. The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Since tangential-flow filtration is an efficient method for concentration and separation of bimolecular by retaining.
Verorab Sanofi Pasteur has been used in rabies prevention since 1985. We investigated if the cost of immunization with PVRV could be further reduced by intradermal immunization. Study of Purified Vero Rabies Vaccine and Rabies Human Diploid Cell Vaccine in a Simulated Rabies Post-exposure Regimen.
A serum-free purified vero cell rabies vaccine is safe and as immunogenic as the reference vaccine Verorab for pre-exposure use in healthy adults. Epub 2013 Mar 16. All patients are alive and well after 1 year.
Side-effects of treatment were negligible. This study has a goal to determine the immunogenicity and safety of SPEEDA and TRCS-SPEEDA SPEEDA which is filled by Queen Saovabha Memorial Institutevs. Listing a study does not mean it has been evaluated by the US.
47 patients with severe exposure were also given 20 IUkg human rabies immune globulin HRIG. We studied the safety and immunogenicity of the TRC-ID regimen with a new CPRV in non-immunized Thai children with possible or proven rabies exposure. If you have a continued risk of exposure to rabies you may need to receive the preventive vaccine series every 2 years.
Antibody responses were monitored by using the rapid fluorescent-focus inhibition test RFFIT and an inhibition enzyme immunoassay INH EIA on days 0 7 28 and 49. A serum-free purified vero cell rabies vaccine is safe and as immunogenic as the reference vaccine Verorab for pre-exposure use in healthy adults. Read our disclaimer for details.
Maximum antibody titers determined using a rapid fluorescent focus inhibition test were 25 IU or 90 days after the first vaccination and were 30 IU on day 1050 after booster vaccination. Purified Vero cell rabies vaccine PVRV contains inactivated and lyophilized Wistar strain of rabies virus grown on Vero cell cultures in fermenters allowing mass cultivation. The purified Vero cell rabies vaccine PVRV.
Primary Hamster Kidney Cell vaccine PHKCV uses the Beijing strain and is inactivated with formalin and adsorbed to aluminum hydroxide. Interval of 21 days had the efficacy of 79 against symptomatic SARS-CoV-2 infection 14 days or more after the second dose. The safety and immunogenicity profile of the WHO-approved two-site intradermal Thai Red Cross regimen modified TRC-ID regimen.
Results from a randomized controlled phase-II trial. It represents a significant saving in vaccine cost and is now established in several developing countries. Thus a consolidated review comparing immunogenicity of PVRV ID regimens during pre.
05 ml PVRV was given intramuscularly on days 0 3 7 14 28 and 91.

Medical Product Alert N 8 2019 Falsified Rabies Vaccines And Anti Rabies Serum

An Overview Of The Immunogenicity And Effectiveness Of Current Human Rabies Vaccines Administered By Intradermal Route Sciencedirect
Https Www Who Int Vaccine Safety Initiative Tools Rabies Vaccine Rates Information Sheet Pdf
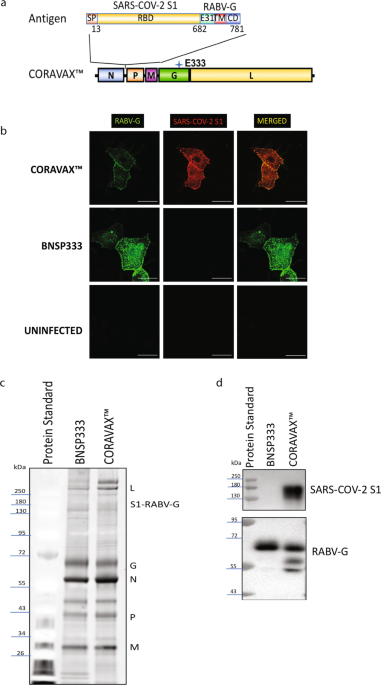
Rabies Virus Based Covid 19 Vaccine Coravax Induces High Levels Of Neutralizing Antibodies Against Sars Cov 2 Npj Vaccines
Https Academic Oup Com Cid Article Pdf 29 1 141 921370 29 1 141 Pdf
Https Www Mdpi Com 2414 6366 5 1 40 Pdf

Pdf Safety And Immunogenicity Of Purified Vero Cell Rabies Vaccine Versus Purified Chick Embryo Cell Rabies Vaccine Using Pre Exposure And Post Exposure Regimen Among Healthy Volunteers In San Lazaro Hospital
Abhayrab Rabies Vaccine United Pharmacies

China Rabies Vaccine Vero Cell For Human Use Freeze Dried China Vaccine Human

Promising Rabies Vaccine For Postexposure Prophylaxis In Developing Countries A Purified Vero Cell Vaccine Produced In China Clinical And Vaccine Immunology

Intradermal Post Exposure Rabies Vaccination With Purified Vero Cell Rabies Vaccine Comparison Of A One Week 4 Site Regimen Versus Updated Thai Red Cross Regimen In A Randomized Non Inferiority Trial In The Philippines Sciencedirect
Abhayrab Full Prescribing Information Dosage Side Effects Mims Philippines

Chromatographically Purified Vero Cell Rabies Vaccine Cprv Biovalys
Https Www Tandfonline Com Doi Pdf 10 1080 14760584 2017 1294068
China Products Of Rabies Vaccine Vero Cell For Human Use High Quality Products Of Rabies Vaccine Vero Cell For Human Use On Bossgoo Com

Intradermal Post Exposure Rabies Vaccination With Purified Vero Cell Rabies Vaccine Comparison Of A One Week 4 Site Regimen Versus Updated Thai Red Cross Regimen In A Randomized Non Inferiority Trial In The Philippines Sciencedirect
Speeda Full Prescribing Information Dosage Side Effects Mims Myanmar
Https Www Mdpi Com 2414 6366 5 1 40 Pdf

Pdf Purified Vero Cell Rabies Vaccine And Human Diploid Cell Strain Vaccine Comparison Of Neutralizing Antibody Responses To Post Exposure Regimens